
Provider Corner
FEATURING:

Fluorescent Imaging Technology Available at Moffitt Cancer Center for Ovarian Cancer Surgery
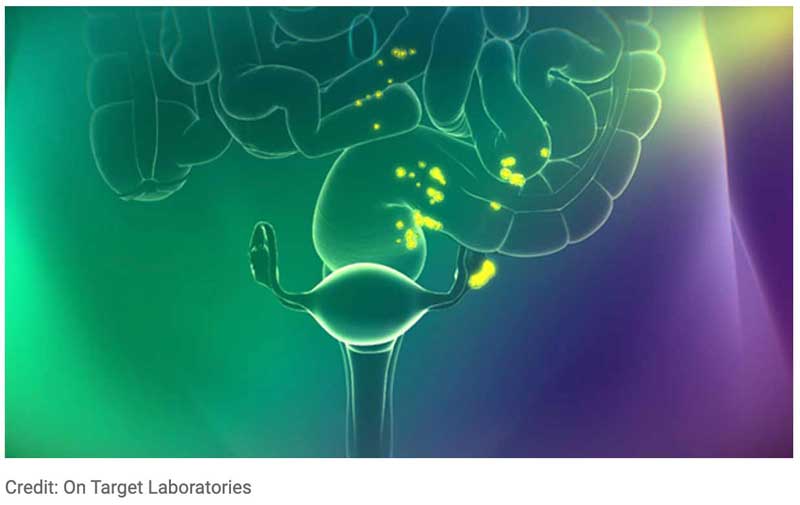 Cytalux™, an FDA-approved optical imaging agent, is now available at Moffitt Cancer Center. This treatment is for adult patients with ovarian cancer and can be used as an adjunct for the intraoperative identification of malignant lesions. Moffitt is the first institution in the country that was part of Phase 2 and 3 trials to offer this treatment therapy.
Cytalux™, an FDA-approved optical imaging agent, is now available at Moffitt Cancer Center. This treatment is for adult patients with ovarian cancer and can be used as an adjunct for the intraoperative identification of malignant lesions. Moffitt is the first institution in the country that was part of Phase 2 and 3 trials to offer this treatment therapy.
Ovarian cancer is the number one cause of gynecologic cancer death in the United States. For women with ovarian cancer, treatment often consists of a combination of cytoreductive surgery and chemotherapy. Now, surgeons will be able to administer the fluorescent imaging technology using the standard I.V. before surgery. Cytalux agent binds to folate receptors that are overexposed in most epithelial ovarian cancer cases and light up intraoperatively under near-infrared light. This helps surgeons to identify malignant lesions that were not preplanned or detected by palpation and normal white light alone.
"The multidisciplinary experts at Moffitt Cancer Center were instrumental in the development of this technology as active investigators in Phase 2 and randomized Phase 3 studies. Our tissue core lab served as their central lab for study. This novel therapy treatment is a significant milestone that will advance the way we detect cancer. We know that survival is better with more cancer that is removed. The removal of more tumors is associated with better survival for patients. We’re excited about the potential of this technology to help patients live longer. We hope to see more patients when they are first diagnosed and need surgery so we can offer this novel treatment. A patient’s best chance is her first chance,” says Gynecologic Program Chair Robert Wenham, MD.
 The Department of Gynecologic Oncology at Moffitt Cancer Center is known for providing world-class, individualized treatment, as well as some of the highest survival rates and improved quality of life. We offer the latest options in diagnostic imaging, laboratory testing, and genetic screening, as well as innovations in surgery using Cytalux, chemotherapy, radiation therapy, and hormone therapy.
The Department of Gynecologic Oncology at Moffitt Cancer Center is known for providing world-class, individualized treatment, as well as some of the highest survival rates and improved quality of life. We offer the latest options in diagnostic imaging, laboratory testing, and genetic screening, as well as innovations in surgery using Cytalux, chemotherapy, radiation therapy, and hormone therapy.
Our robust clinical trials program, in which some of our experts are leading investigators, provides patients with unique opportunities to benefit from promising new treatments before those options are made widely available in other settings.
Provider Corner

Cleveland Clinic Performs World’s First Implant of Combined Heart Failure Therapies

Heart Transplant Innovation: New ‘heart in a box’ technology provides gift of life to Arizona man, as couple prepares to celebrate 50-year anniversary

The future of Wilms tumor therapies: Q&A with Jeffrey Dome, M.D., Ph.D.
Subscribe to our Newsletter
Our SOlutions
Contact Us
Phone Number: +1 (954) 370-6404
Toll-Free (in the U.S. & Canada):
800-682-6065
Email: info@gmmi.com
2024 © All Rights Reserved.
Code of Conduct | GDPR | PIPEDA | Terms of Use | Privacy Policy | California Privacy Notice | Personal Data Access Form | Responsible Data Leak Disclosure Form



